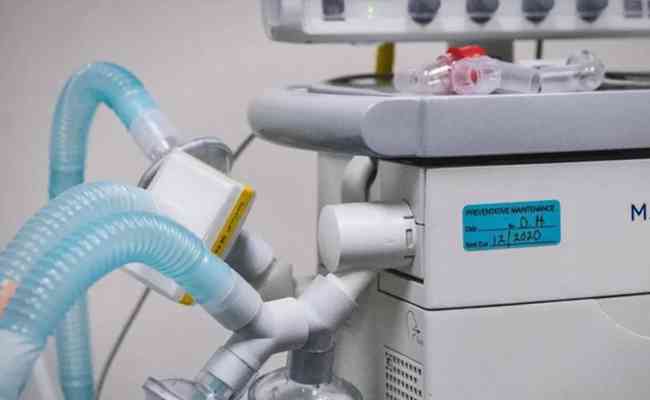
Prisma Health develops FDA-authorized 3D-printed device
By MYBRANDBOOK
The ultimate winner is going to be the technology, in era of global health disruption with the COVID 19 infection going to touch 6.5 Lakh ,where US is the front runner,followed by China and Italy.
There is huge shortage of ventilators for U.S. hospitals is likely already a crisis, That's why a simple piece of hardware newly approved by the FDA for emergency use and available free via source code and 3D printing for hospitals, might be a key ingredient in helping minimize the strain on front-line response efforts. Devices cleared under FDA Emergency Use Authorization (EUA) like this one are fully understood to be prototypes, and the conditions of their use includes a duty to report the results of how they perform in practice.
The Prisma Health VESper is a deceptively simple-looking three-way connector that expands use of one ventilator to treat up to four patients simultaneously. The device is made for use with ventilators that comply to existing ISO standard ventilator hardware and tubing, and allows use of filtering equipment to block any possible transmission of viruses and bacteria.
VESper works in device pairs, with one attached to the intake of the ventilator, and another attached to the return. They also can be stacked to allow for treatment of up to four patients at once - provided the patients require the same clinical treatment in terms of oxygenation, including the oxygen mix as well as the air pressure and other factors.
This was devised by Dr. Sarah Farris, an emergency room doctor, who shared the concept with her husband Ryan Farris, a software engineer who developed the initial prototype design for 3D printing. Prisma Health is making the VESper available upon request via its printing specifications, but it should be noted that the emergency use authorization under which the FDA approved its use means that this is only intended effectively as a last-resort measure - for institutions where ventilators approved under established FDA rules have already been exhausted, and no other supply or alternative is available in order to preserve the life of patients.
In addition to offering the plans for in-house 3D printing, Prisma Health has sourced donations to help print units for healthcare facilities that don’t have access to their own 3D printers.


Legal Battle Over IT Act Intensifies Amid Musk’s India Plans
The outcome of the legal dispute between X Corp and the Indian government c...

Wipro inks 10-year deal with Phoenix Group's ReAssure UK worth
The agreement, executed through Wipro and its 100% subsidiary,...

Centre announces that DPDP Rules nearing Finalisation by April
The government seeks to refine the rules for robust data protection, ensuri...
Home Ministry cracks down on PoS agents in digital arrest scam
Digital arrest scams are a growing cybercrime where victims are coerced or ...


Icons Of India : Deepak Sharma
Deepak Sharma spearheads Schneider Electric India. He brings with him ...

Icons Of India : NATARAJAN CHANDRASEKARAN
Natarajan Chandrasekaran (Chandra) is the Chairman of Tata Sons, the h...

Icons Of India : CP Gurnani
Former Managing Director and CEO of the well-known IT service company ...


NIC - National Informatics Centre
NIC serves as the primary IT solutions provider for the government of ...

NSE - National Stock Exchange
NSE is the leading stock exchange in India....

NPCI - National Payments Corporation of India
NPCI is an umbrella organization for operating retail payments and set...


Indian Tech Talent Excelling The Tech World - Lal Karsanbhai, President & CEO, Emerson
Lal Karsanbhai, President and CEO of Emerson, assumed the leadership i...

Indian Tech Talent Excelling The Tech World - Satya Nadella, Chairman & CEO- Microsoft
Satya Nadella, the Chairman and CEO of Microsoft, recently emphasized ...

Indian Tech Talent Excelling The Tech World - REVATHI ADVAITHI, CEO- Flex
Revathi Advaithi, the CEO of Flex, is a dynamic leader driving growth ...
 of images belongs to the respective copyright holders
of images belongs to the respective copyright holders