Serum Institute of India will play pivotal role in development of COVID-19 vaccines
By MYBRANDBOOK
Serum Institute of India (SII) is the world’s largest vaccine producer in terms of volume. SII is currently working on three candidates for COVID-19, including the testing of its recombinant-BCG vaccine in Phase 3 clinical trials at several locations across India. With these, SII continues to play a pivotal role in the development and commercialization of COVID-19 vaccines, says GlobalData, a leading data and analytics company.
Krishna Srinivasaraghavan, Pharma Analyst at GlobalData, says: “Serum Institute of India has invested US$100m for setting up a COVID-19 vaccine manufacturing unit in India. SII is highly active on three fronts – development, partnerships and manufacturing capabilities to supply COVID-19 vaccines.”
Earlier, New York Institute of Technology (NYIT) researchers highlighted that countries without universal policies of BCG vaccination have been more severely affected compared to countries with universal and long-standing BCG policies. It is important to note that BCG vaccination is part of the national immunization program in India and the majority of the Asian countries.
Vakzine Projekt Management GmbH (VKM), in which SII is the majority stake holder, has initiated a Phase 3 trial of VPM1002 (BCG vaccine) in May 2020, involving 1,200 healthcare professionals globally and around 6,000 high-risk subjects in India (NCT04387409 and CTRI/2020/04/024749). It is expected to be completed in April 2021.
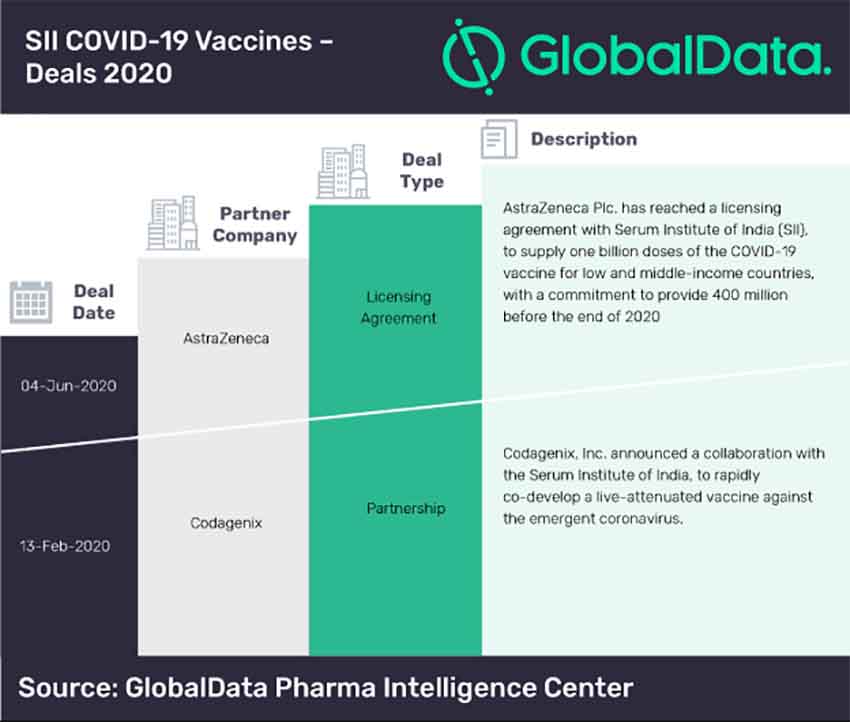
According to GlobalData’s Pharma Intelligence Center, overall, 19 trials are being conducted globally at various locations to test the use of BCG vaccine against COVID-19, especially focused on healthcare workers. These include a Phase 3 ongoing trial being conducted by Murdoch Children’s Research Institute (BRACE trial) in Australia (NCT04327206), which is expected to enrol around 10,000 subjects and complete by October 2020. Trials are also being planned in India by JIPMER (CTRI/2020/04/024833) in Phase III and Haffkine Institute (CTRI/2020/05/025013) to test a BCG vaccine in Phase II for COVID-19.
Srinivasaraghavan concludes: “BCG vaccinnation is already part of the compulsory schedule for children in India. If the BCG trials for COVID-19 are successful, the vaccine can be extended to a wider population in the fight against the pandemic effectively and save lives. Moreover, SII would be able to scale-up the production of BCG vaccine quickly in India.”


Legal Battle Over IT Act Intensifies Amid Musk’s India Plans
The outcome of the legal dispute between X Corp and the Indian government c...

Wipro inks 10-year deal with Phoenix Group's ReAssure UK worth
The agreement, executed through Wipro and its 100% subsidiary,...

Centre announces that DPDP Rules nearing Finalisation by April
The government seeks to refine the rules for robust data protection, ensuri...
Home Ministry cracks down on PoS agents in digital arrest scam
Digital arrest scams are a growing cybercrime where victims are coerced or ...


Icons Of India : Kumar Mangalam Birla
Aditya Birla Group chairman Kumar Mangalam Birla recently made a comeb...

Icons Of India : Dr. Arvind Gupta
Arvind Gupta is the Head and Co-Founder of the Digital India Foundatio...

Icons Of India : MUKESH D. AMBANI
Mukesh Dhirubhai Ambani is an Indian businessman and the chairman and ...


NPCI - National Payments Corporation of India
NPCI is an umbrella organization for operating retail payments and set...

TCIL - Telecommunications Consultants India Limited
TCIL is a government-owned engineering and consultancy company...

CSC - Common Service Centres
CSC initiative in India is a strategic cornerstone of the Digital Indi...


Indian Tech Talent Excelling The Tech World - Soni Jiandani, Co-Founder- Pensando Systems
Soni Jiandani, Co-Founder of Pensando Systems, is a tech visionary ren...

Indian Tech Talent Excelling The Tech World - NEAL MOHAN, CEO - Youtube
Neal Mohan, the CEO of YouTube, has a bold vision for the platform’s...

Indian Tech Talent Excelling The Tech World - Steve Sanghi, Executive Chair, Microchip
Steve Sanghi, the Executive Chair of Microchip Technology, has been a ...
 of images belongs to the respective copyright holders
of images belongs to the respective copyright holders